This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
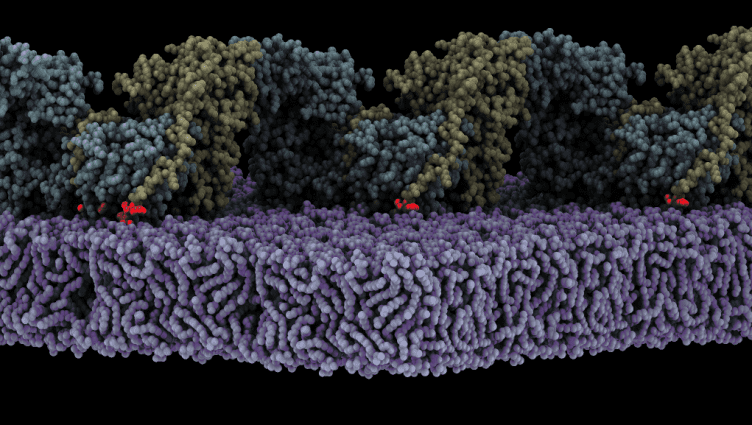 Assembly of oligomeric FAK (yellow/cyan) on the membrane (purple) triggers autophosphosphorylation. Shown is a state where the autophosphorylation site (red glow) is bound to the active site of the FAK kinase. /CIB-CSIC
Assembly of oligomeric FAK (yellow/cyan) on the membrane (purple) triggers autophosphosphorylation. Shown is a state where the autophosphorylation site (red glow) is bound to the active site of the FAK kinase. /CIB-CSIC
The uncovered mechanism is key for cancer cell invasion promoted by the FAK protein
A research team led by Daniel Lietha has just published in The EMBO Journal the mechanistic details of the activation of the Focal Adhesion Kinase (FAK) on lipid membranes. Lietha started this research during his work at the Spanish National Cancer Research Centre (CNIO) and has culminated it in his current institution, the Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC).
FAK is a key protein ensuring controlled cell adhesion, proliferation, migration and survival which in cancer is often responsible for aberrant cell invasion leading to metastatic cancers. In the cytosol, FAK adopts an autoinhibited state but is activated upon recruitment into focal adhesions, yet how this occurs or what induces structural changes was unknown.
Lietha’s group have demonstrated that FAK is activated when it is localised to the cell membrane where it interacts with specific phosphoinositide lipids. Now, the high-resolution structure of an oligomeric form of FAK bound to a lipid membrane has been obtained using Cryo-Electron Microscopy. The analysis of the structure shows that initial binding of FAK to the membrane causes steric clashes that release the kinase domain from autoinhibition, allowing it to undergo a large conformational change and interact itself with the membrane in an orientation that places the active site towards the membrane.
The structure also reveals that several interfaces align in the rearranged conformation to allow oligomerization of FAK on the membrane with a key phosphorylation site exposed, leading to autophosphorylation and, in turn, activation of FAK. Molecular dynamics simulations were carried out to understand the mechanism and dynamics of the process of autophosphorylation and subsequent activation on the membrane.
To validate the computational model, different mutants of FAK have been generated carrying mutations at the observed interfaces. Extensive biochemical experiments have been carried out to evaluate how the different mutations affect lipid binding, FAK autophosphorylation and activation. Moreover, how the mutations affect FAK function in cancer cells was also studied revealing that the uncovered mechanism is key for cancer cell invasion and proliferation.
This work is the result of an international collaboration between the CIB Margarita Salas and researchers at the Spanish National Cancer Research Centre (Madrid, Spain), Center for Cellular Imaging and NanoAnalytics, Biozentrum (University of Basel, Switzerland), Cancer Research UK Edinburgh Centre, Institute of Genetics and Molecular Medicine (University of Edinburgh, United Kingdom), Heidelberg Institute for Theoretical Studies and Interdisciplinary Center for Scientific Computing (Heidelberg, Germany).
The research has been funded by the Spanish Ministry of Science and Innovation, the National Institute of Health Carlos III, the Spanish Ministry of Education, Culture and Sports, the Werner-Siemens Foundation, the University of Basel, the Swiss National Science Foundation, Cancer Research UK, the Klaus Tschira Foundation, the Deutsche Forschungsgemeinschaft, the State of Baden-Wuertenberg, the Carl Zeiss Foundation, the European Regional Development Fund, the European Social Fund, the Spanish National Research Council, and the Autonomous Region of Madrid.
Reference article
Structural basis of Focal Adhesion Kinase activation on lipid membranes. Iván Acebrón et al (EMBO J, 2020). DOI: https://www.embopress.org/doi/10.15252/embj.2020104743
